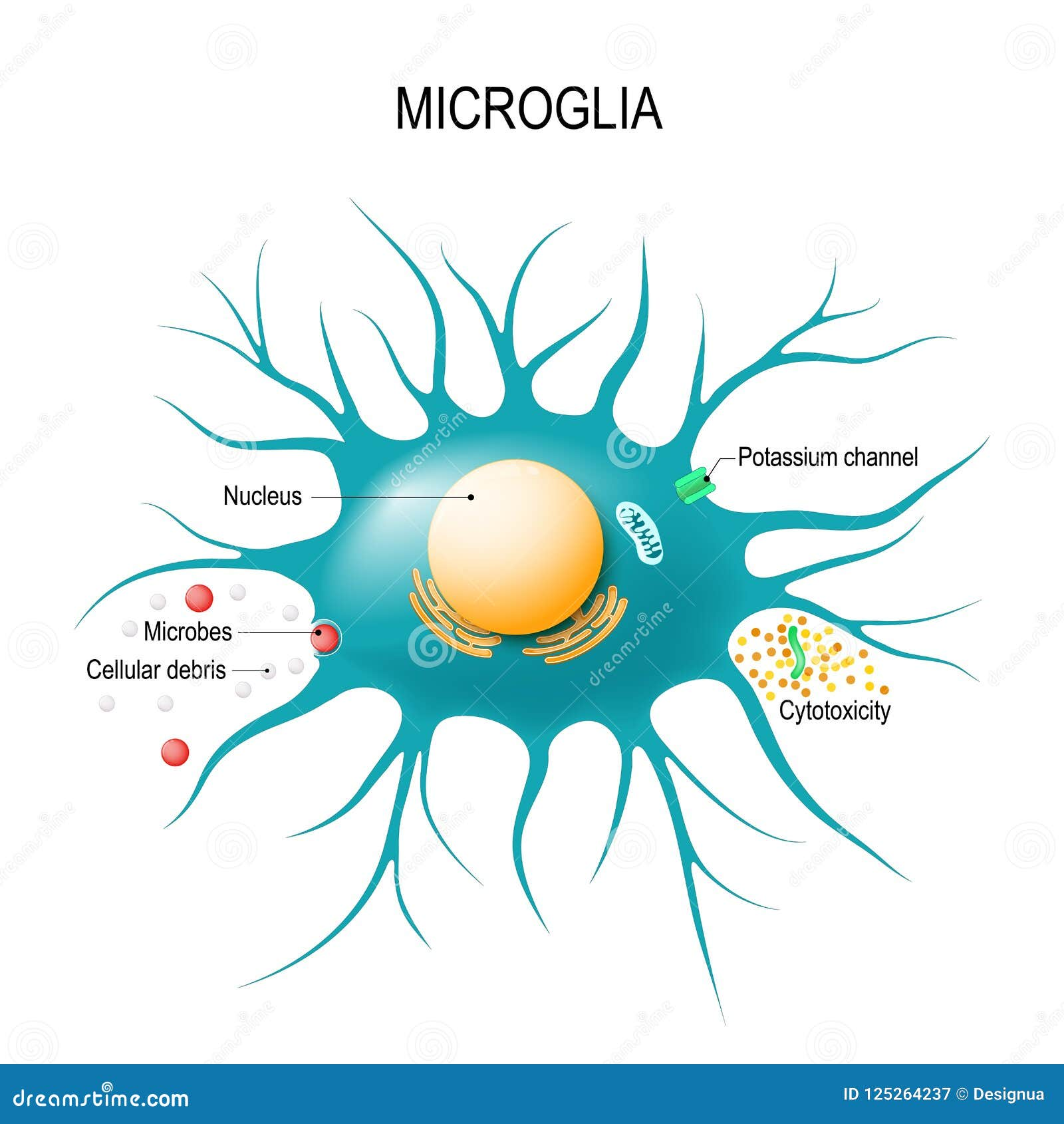
Microglial cells are critical players in the brain’s immune system, acting as vigilant guardians that monitor for injury and disease. These specialized cells are instrumental in maintaining cognitive function, particularly through their roles in synaptic pruning—a process that helps eliminate unused or damaged synapses between neurons. However, recent research led by neuroscientist Beth Stevens has revealed that dysregulation of microglial activity can contribute to the progression of neurodegenerative diseases, including Alzheimer’s. By investigating these powerful immune cells, Stevens and her team are paving the way for innovative treatments and potential biomarkers that could revolutionize Alzheimer’s research. Their findings underscore the importance of understanding microglial function in hopes of improving care for millions affected by these devastating conditions.
Often referred to as the brain’s resident immune cells, microglia play an essential role in maintaining neurological health by responding to various challenges that may arise within the central nervous system. These cells embark on a crucial journey of synapse remodeling, ensuring that neuronal connections are optimized for healthy brain function. However, when their activity goes awry, as seen in conditions like Alzheimer’s and Huntington’s diseases, the consequences can be dire. Pioneering researchers like Beth Stevens are at the forefront of exploring the implications of microglial behavior, shedding light on how these immune agents can be both protectors and potential contributors to neurodegeneration. Through their work, they aim to unlock new avenues for effective treatments and interventions for those struggling with neurodegenerative illnesses.
Understanding the Role of Microglial Cells in Brain Health
Microglial cells are a crucial part of the brain’s immune system, constantly on patrol for potential threats such as pathogens or damaged cells. They are akin to the guardians of the central nervous system, providing not only protection but also support critical for brain health. These specialized cells engage in synaptic pruning, the process of removing unnecessary or weak synapses, which is vital for maintaining efficient neuronal communication and healthy brain function. In context of Alzheimer’s research, the proper functioning of microglia is imperative, as their malfunction can lead to neurodegenerative diseases by failing to clear out synaptic debris or by excessively pruning synapses.
Recent studies led by researchers like Beth Stevens have highlighted the fascinating dual role of microglial cells. While they possess the ability to protect the brain and ensure healthy neuronal connections through synaptic pruning, they can also contribute to the pathology of diseases when their activities go awry. For instance, altered microglial activity has been linked to increased synaptic loss in Alzheimer’s patients, exacerbating the disease. Understanding the precise mechanisms by which microglial cells operate is crucial for developing innovative treatments aimed at modulating their behavior to prevent or mitigate neurodegeneration.
The Impact of Aberrant Synaptic Pruning on Neurodegenerative Diseases
Aberrant synaptic pruning is increasingly recognized as a contributing factor to various neurodegenerative diseases, including Alzheimer’s and Huntington’s. In healthy brains, microglial cells carefully remove excess synapses during development, promoting optimal neural circuitry and communication. However, when microglia become overactive or misregulated, this pruning process can spiral out of control, leading to significant synaptic loss and cognitive decline. Beth Stevens’ research emphasizes how understanding these mechanisms is crucial in the quest to identify novel biomarkers for early detection of neurodegenerative disorders.
The ramifications of disruptive synaptic pruning extend beyond simply losing neuronal connections; they also interfere with the brain’s overall immune response. New findings suggest that preventing harmful pruning activities may open pathways for therapeutic interventions aimed at restoring normal synaptic function. This highlights the necessity for continued exploration in the realm of Alzheimer’s research, as insights gained can lay the groundwork for treatments that target microglial behavior, ultimately altering the disease’s trajectory for millions suffering from these ailments.
Beth Stevens and the Evolution of Alzheimer’s Research
Dr. Beth Stevens represents a shift in Alzheimer’s research, focusing on the immune functions of the brain rather than just the direct effects of amyloid plaques and tau tangles. Her pioneering work on microglial cells has shown that understanding the immune system’s involvement in neurodegeneration could yield transformative breakthroughs in treatment. Through her dedication to elucidating the role of these cells in synaptic pruning and maintenance, Stevens highlights the potential for a new understanding of how neurodegenerative diseases manifest and progress.
The recognition of Stevens’ contributions to neuroscience, such as her receipt of the MacArthur Fellowship in 2015, underscores the significance of innovative research in reshaping our understanding of diseases like Alzheimer’s. As she pursues further investigations into the immune-related mechanisms at play, Stevens’ work may pave the way for new strategic approaches in treating neurodegenerative diseases and improving the lives of those affected by them.
Synaptic Pruning and Its Implications in Neurodevelopment
Understanding synaptic pruning is essential in appreciating how the brain develops and maintains its intricate networks. This dynamic process, driven by microglial cells, ensures that only the most efficient connections between neurons remain, allowing for seamless communication and cognitive function. During periods of significant brain development, such as adolescence, the role of synaptic pruning becomes particularly critical, as the brain undergoes substantial structural changes.
Moreover, aberrant or excessive synaptic pruning during these development phases can set the stage for future neuropsychiatric disorders. Research indicates that disruptions in this process might be linked to conditions such as schizophrenia and autism. By studying microglia and their influence on synaptic pruning, scientists hope to uncover the mechanisms behind these disorders, enhancing our ability to create early interventions and potentially prevent the onset of neurodevelopmental issues.
The Future of Alzheimer’s Treatments Hinges on Microglial Research
The future of effective Alzheimer’s treatments may very well rest on the advancements made in microglial research, particularly in how these cells regulate synaptic pruning. As scientists like Beth Stevens uncover the links between microglial activity and the onset of neurodegenerative diseases, new therapeutic strategies can be conceived. These could involve drugs that enhance the protective roles of microglia or block their harmful pruning actions, tailoring treatments to the individual needs of patients.
Additionally, exploring the potential of microglial-targeted therapies offers promise not only for treating Alzheimer’s but for addressing a wider array of neurodegenerative conditions. By focusing on the immune system of the brain, researchers are poised to shift treatment paradigms, moving away from merely symptomatic therapies to more holistic approaches that could modify disease progression and improve quality of life for those affected.
The Importance of Federal Funding in Alzheimer’s Research
Federal funding has played a pivotal role in advancing Alzheimer’s research, allowing scientists like Beth Stevens to pursue groundbreaking inquiries into the workings of microglial cells. Such funding supports essential studies that unravel the complexities of neurodegenerative diseases, offering researchers the resources needed to explore innovative hypotheses. Without financial backing from institutions like the National Institutes of Health, many significant discoveries in the realm of brain health and immune responses might remain undiscovered.
Investments in basic science forge pathways for new treatments and interventions, illuminating areas previously thought to be abyssal. As the understanding of the brain’s immune system and microglia’s role deepens, ongoing support from federal entities will be crucial in translating these findings into viable therapeutic options for millions at risk for or suffering from Alzheimer’s disease.
Microglial Cells: Key Players in Neurodegenerative Disease Mechanisms
Microglial cells are emerging as key players in the mechanisms underlying various neurodegenerative diseases. Their role extends beyond maintenance and protection; abnormal microglial activation can contribute to inflammatory processes that exacerbate conditions such as Alzheimer’s disease and other related disorders. Research indicates that the actions of microglia can significantly influence neurodegenerative pathways, making them critical targets for future therapies aimed at modulating immune responses in the brain.
As scientists actively investigate the dual nature of microglia, understanding how they can be harnessed or mitigated is essential. This exploration will help clarify whether enhancing their protective roles might help stave off neurodegeneration while also addressing the inflammatory aspects that may drive these diseases forward in affected individuals. Such insights could revolutionize therapeutic strategies, shifting the focus toward the immune response as a vital component in managing neurodegenerative conditions.
Innovations in Biomarkers Derived from Microglial Research
Innovations in biomarkers linked to microglial activity could vastly improve the early diagnosis of neurodegenerative conditions such as Alzheimer’s. Understanding how microglial cells respond to synaptic changes offers the potential to identify specific proteins or signaling pathways that are altered in disease states. Beth Stevens’ work emphasizes that by pinpointing these biomarkers, researchers may be able to develop reliable diagnostic tools that detect Alzheimer’s even before clinical symptoms manifest.
The advent of such biomarkers would not only enable earlier interventions but also facilitate the monitoring of disease progression and treatment efficacy. As research continues to unfold the complex roles of microglia in neurodegeneration, scientists can aspire to create comprehensive profiles that allow for more personalized and proactive care for individuals facing the challenges of these serious conditions.
The Future of Neurodegenerative Disease Research: Challenges and Opportunities
As the field of neurodegenerative disease research progresses, challenges and opportunities remain at the forefront of scientific inquiry. The interplay between genetics, environmental factors, and the immune system makes diseases like Alzheimer’s incredibly complex. Researchers must navigate these intricacies while remaining focused on discovering actionable insights that can lead to effective treatments. The work of innovators like Beth Stevens highlights the power of curiosity-driven research to unravel these complexities and contribute to transformative change.
Moreover, as understanding deepens regarding the roles of microglial cells and other immune components in brain health, opportunities for cross-disciplinary collaboration will grow. By uniting insights from genetics, immunology, and neurology, the scientific community can forge ahead in designing novel therapeutic strategies, potentially reshaping the landscape of neurodegenerative disease management and care.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells serve as the brain’s immune system, patrolling for illness and injury. In Alzheimer’s research, they are crucial as they help clear dead cells and prune synapses, processes that can become dysregulated and contribute to the progression of Alzheimer’s disease and other neurodegenerative diseases.
How do microglial cells contribute to synaptic pruning in neurodegenerative diseases?
Microglial cells play a key role in synaptic pruning, which is essential for normal brain development. However, in neurodegenerative diseases such as Alzheimer’s and Huntington’s, these cells can engage in aberrant pruning. This improper synaptic elimination can disrupt neural circuits and exacerbate disease progression.
Who is Beth Stevens and what is her significance in microglial cell research?
Beth Stevens is a prominent neuroscientist and NIH-supported investigator focused on Alzheimer’s disease. Her research has transformed our understanding of microglial cells, emphasizing their role in the brain’s immune response and their impact on synaptic pruning, leading to significant advances in neurodegenerative disease treatment.
What impact do microglial cells have on the brain’s immune system?
As integral components of the brain’s immune system, microglial cells are responsible for monitoring brain health, clearing debris from damaged cells, and modulating neuronal circuits through synaptic pruning, which is vital for maintaining cognitive function and preventing neurodegenerative diseases.
Can dysregulation of microglial cells lead to neurodegenerative diseases?
Yes, dysregulated microglial cells can lead to neurodegenerative diseases such as Alzheimer’s. When these cells malfunction, they can excessively prune synapses or fail to clear cellular debris properly, contributing to neuronal damage and disease onset.
What biomarker discoveries are linked to microglial cells in the context of Alzheimer’s?
Recent research on microglial cells has led to potential biomarker discoveries that may help detect Alzheimer’s disease early. Understanding how microglia respond to neurodegeneration can provide insights for developing treatments that target these pathways.
How does microglial activity relate to synaptic health in neurodegenerative diseases?
Microglial activity is crucial for maintaining synaptic health, as these cells help sculpt neural connections through synaptic pruning. In neurodegenerative diseases, abnormal microglial activity can lead to the loss of crucial synapses, negatively impacting cognitive function.
Why is the study of microglial cells essential for developing treatments for neurodegenerative diseases?
Studying microglial cells is essential because they influence inflammation, immune responses, and synaptic pruning in the brain. Insights from microglial research can inform strategies for developing therapies that modify disease processes in conditions like Alzheimer’s and ultimately improve patient outcomes.
| Key Point | Details |
|---|---|
| Microglial Cells Role | Act as the brain’s immune system, monitoring for illness and injury. |
| Pruning Function | Clear dead or damaged cells and prune synapses for healthy neuron communication. |
| Impacts of Aberrant Pruning | Malfunctioning pruning can contribute to Alzheimer’s, Huntington’s, and other neurodegenerative disorders. |
| Research Foundation | The work is built on basic science and funded largely by federal agencies, notably NIH. |
| Future Implications | Findings may lead to new biomarkers and treatments for diseases affecting millions in the U.S. |
| Stevens’ Approach | Driven by curiosity in understanding microglial functions and their relationship to brain health. |
Summary
Microglial cells play a crucial role in protecting brain health by acting as the immune system of the brain. Their continuous monitoring helps detect and manage signs of injury or disease, which is vital for maintaining neurological function. Understanding their complex interactions and the consequences of their malfunction is essential for developing effective treatments for neurodegenerative diseases like Alzheimer’s. The ongoing research led by experts like Beth Stevens is paving the way for new therapeutic strategies that can significantly improve the lives of millions affected by these disorders.