Alzheimer’s disease research has become a pivotal area of focus for scientists worldwide, as they seek innovative solutions to combat this devastating neurodegenerative disorder. Led by trailblazers like neuroscientist Beth Stevens, whose work centers on microglial cells—the brain’s immune defenders—this field is making significant strides towards understanding the underlying mechanisms of Alzheimer’s. At Boston Children’s Hospital, Stevens and her team are exploring how improper pruning of synapses by microglia can contribute to the progression of Alzheimer’s and related diseases. Their groundbreaking discoveries not only promise to enhance our knowledge of these conditions but also pave the way for potential Alzheimer’s treatments that could improve the lives of millions. As the aging population increases the prevalence of Alzheimer’s in the United States, research efforts have never been more critical.
Exploring advancements in the study of Alzheimer’s disease encompasses a variety of approaches aimed at understanding this complex condition. Researchers like Beth Stevens are investigating the roles of neuroinflammation and cellular interactions within the brain, paying close attention to microglial activity. Located at prestigious institutions like Boston Children’s Hospital, the Stevens Lab is at the forefront of developing new methodologies to unveil the intricacies of Alzheimer’s and other neurodegenerative diseases. Through their work, the team aims to identify innovative biomarkers and therapeutic strategies that could transform current care practices. As the need for effective Alzheimer’s treatments escalates, the scientific community’s commitment to unraveling these mysteries continues to grow.
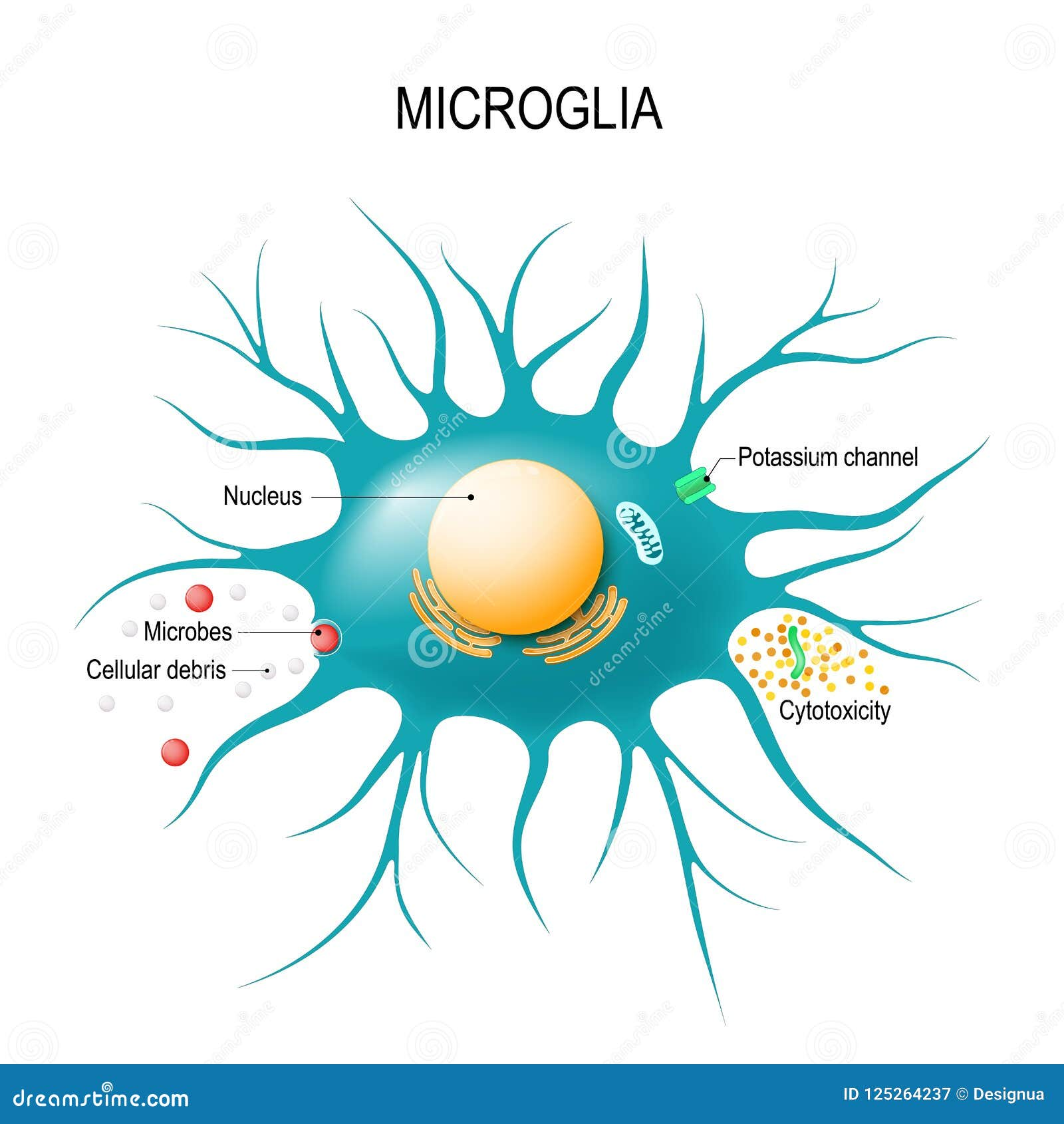
Understanding Microglial Cells in Alzheimer’s Disease
Microglial cells play a pivotal role in the brain, acting as the first line of defense for the central nervous system. These immune cells constantly survey their environment, responding to injury and disease by clearing away debris and damaged cells. In the context of Alzheimer’s disease, recent research led by Beth Stevens and her team at Boston Children’s Hospital has revealed that these cells may not always perform their task correctly, leading to faulty synaptic pruning. This aberrant pruning can exacerbate the neurodegenerative processes underlying Alzheimer’s, providing crucial insights into potential therapeutic targets.
Stevens’ groundbreaking research has shifted the understanding of the relationship between microglial function and neurodegenerative diseases. By highlighting the dual role of microglia in both protecting and potentially harming neuronal health, the team has opened avenues for innovative Alzheimer’s treatments. Efforts to better understand how these cells can be harnessed or modulated could lead to breakthroughs in therapies that not only treat symptoms but also address the root causes of Alzheimer’s disease.
The Implications of Aberrant Pruning in Neurodegenerative Diseases
Aberrant synaptic pruning, as revealed in Beth Stevens’ studies, extends beyond Alzheimer’s disease, implicating conditions such as Huntington’s disease and other neurodegenerative disorders. This insight emphasizes the need for a holistic understanding of brain health, where the immune functions of microglia are considered integral to the development of neurodegenerative illnesses. The Stevens Lab’s findings could pave the way for novel biomarkers, facilitating earlier detection of such diseases and potentially improving patient outcomes.
The significance of these discoveries cannot be understated, especially as the population ages and the incidence of Alzheimer’s and similar diseases rises dramatically. With projections suggesting that the number of Alzheimer’s cases could double by 2050, understanding the role of microglial cells becomes vital for public health initiatives. The implications are clear: establishing therapeutic strategies that target the underlying mechanisms of synaptic pruning could revolutionize the management of neurodegenerative diseases.
Innovative Research Approaches at Boston Children’s Hospital
At Boston Children’s Hospital, the innovative research led by Beth Stevens exemplifies the intersection of basic science and clinical application. By leveraging genetic and molecular techniques, Stevens’ team investigates the pathways that microglial cells utilize to interact with neurons. This approach not only enhances the fundamental understanding of brain immune responses but also provides a framework for developing new therapeutic strategies aimed at Alzheimer’s treatments. As Stevens noted, many discoveries stem from curiosity-driven research, proving that exploration and inquiry can lead to significant advancements in the field.
The collaborative environment at Boston Children’s Hospital and the Broad Institute has fostered a culture of interdisciplinary research. By bringing together scientists from various fields, the lab is accelerating the pace of discovery. The research findings have substantial implications not just for Alzheimer’s disease but also for other neurodegenerative disorders, offering hope for better diagnostic tools and effective therapies that can ultimately improve the quality of life for millions of individuals affected by these relentless diseases.
Driving Progress in Alzheimer’s Disease Research
Research in Alzheimer’s disease has always required a multi-faceted approach, integrating basic science with applied research. The work of Beth Stevens illustrates this blend perfectly; her research focuses on understanding the fundamental roles of microglial cells while also addressing how these findings can translate to treatment options for patients. This ongoing effort provides a new lens through which Alzheimer’s treatments can be viewed, emphasizing the importance of immune responses in the brain as potential therapeutic targets.
As new discoveries accumulate, the urgency to translate this knowledge into actionable solutions grows. The increasing prevalence of Alzheimer’s, coupled with rising healthcare costs, underscores the need for innovative research endeavors. Stevens’ lab is at the forefront of this effort, leveraging its groundbreaking findings not only to develop new medical interventions but also to inspire future generations of scientists dedicated to unraveling the complexities of neurodegenerative diseases.
Federal Funding and Its Impact on Alzheimer’s Research
The success of Beth Stevens’ research can be attributed in part to the crucial support from federal funding bodies like the National Institutes of Health (NIH). This financial backing has enabled her lab to explore complex questions concerning microglial cell behavior and its link to Alzheimer’s disease. Stevens emphasizes that the foundational research, driven by curiosity and supported by federal grants, paves the way for significant breakthroughs in understanding and treating neurodegenerative diseases.
However, the nature of scientific research means that not all endeavors lead directly to tangible outcomes; sometimes, they lead to unexpected discoveries that can open new avenues for therapeutic strategies. This unpredictability is inherent in the scientific process, and federal funding plays a vital role in allowing researchers the freedom to follow these scientific leads without the immediate pressure for results. By investing in basic science, funding agencies contribute significantly to advancements in Alzheimer’s treatments and diagnostics.
Collaboration in Alzheimer’s Disease Research
The fight against Alzheimer’s disease is a global challenge that requires collaboration across various scientific disciplines and institutions. In Stevens’ lab at Boston Children’s Hospital, collaboration is at the heart of their research strategy. By working closely with colleagues at institutions such as the Broad Institute, they are harnessing a wide array of expertise to tackle the multifaceted problems presented by neurodegenerative diseases. Such collaborative efforts ensure that projects benefit from diverse perspectives and a wealth of knowledge, fostering innovation and creativity in finding solutions.
Moreover, collaboration extends beyond academia; partnerships with pharmaceutical companies and biotech firms are essential for translating laboratory findings into real-world applications. These collaborations can speed up the process of drug development and ensure that promising Alzheimer’s treatments move swiftly through clinical trials. By bridging the gap between basic research and clinical practice, scientists like Stevens are making significant strides toward combating Alzheimer’s disease through innovative therapies.
Future Directions in Neurodegenerative Disease Research
Looking ahead, the field of neurodegenerative disease research is poised for significant advancements. Researchers like Beth Stevens are exploring various aspects of microglial function to better understand their role in Alzheimer’s disease and beyond. As technologies continue to evolve, the ability to dissect cellular interactions and molecular mechanisms within the brain is becoming increasingly refined, leading to more targeted and effective treatments that could ultimately alter the landscape of Alzheimer’s care.
Additionally, the focus on developing new biomarkers will revolutionize early diagnosis and intervention strategies. Understanding the timing and nature of microglial activity could provide critical insights into when and how neurodegenerative diseases like Alzheimer’s manifest. This knowledge could enable clinicians to administer treatments at earlier stages of disease progression, potentially slowing or even reversing the course of these conditions. The future of Alzheimer’s disease research looks promising, with the potential for a transformative impact on patient care.
Advancements in Alzheimer’s Treatments
The exploration of microglial function has opened new doors for the development of Alzheimer’s treatments. By identifying pathways that regulate microglial behavior, researchers at Boston Children’s Hospital are working on therapeutic interventions that can enhance microglial efficiency in clearing pathology associated with Alzheimer’s. These innovative approaches aim not just to manage the symptoms but to address the underlying processes contributing to neurodegeneration, offering hope for improved patient outcomes.
Furthermore, continued research into the mechanisms of microglial action is critical for creating medications that can modify disease progression. As our understanding of these immune cells deepens, the potential for targeted therapies grows, leading to a new era in Alzheimer’s treatment. The commitment to driving progress in this area is a testament to the dedication of scientists like Beth Stevens, whose work exemplifies the ongoing battle against the epidemic of Alzheimer’s disease.
The Role of Basic Science in Translational Research
Basic science is often the backbone of translational research, laying the groundwork for practical applications in medicine. The investigations into microglial behavior conducted by Stevens and her team are excellent examples of how foundational research can lead to real-world impacts on Alzheimer’s disease. By understanding the basic biological processes that underpin neurodegeneration, researchers can develop strategies that lead to more effective treatments aimed at alleviating the burden of Alzheimer’s on individuals and society.
This emphasis on fundamental research highlights the necessity of continued investment in the scientific endeavors that may seem removed from immediate clinical application. The insights gained from such work frequently provide the key to unlocking more effective therapies, especially in complex disorders like Alzheimer’s. As we strive to better understand the cellular dynamics of neurodegenerative diseases, the commitment to basic science will remain integral to advancing Alzheimer’s research and improving patient care.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are crucial in Alzheimer’s disease research as they represent the brain’s immune system. They actively patrol for signs of illness or injury, clearing out dead cells, and pruning synapses. In Alzheimer’s, aberrant pruning by these cells can lead to cognitive decline, making them a key focus for developing new treatments.
How is Beth Stevens contributing to Alzheimer’s disease research at Boston Children’s Hospital?
Beth Stevens is leading pioneering research on microglial cells at Boston Children’s Hospital, focusing on how these cells affect neurodegenerative diseases, including Alzheimer’s. Her work has illuminated the role of aberrant pruning in Alzheimer’s and has opened avenues for potential biomarkers and therapies to enhance early detection and treatment.
What discoveries have stemmed from the study of microglial cells in relation to neurodegenerative diseases?
The study of microglial cells has uncovered their significant role in neurodegenerative diseases like Alzheimer’s. Research indicates that improper pruning of synapses by these cells contributes to the progression of these diseases, leading to novel therapeutic strategies and potential new biomarkers for early diagnosis.
Why is research on microglial cells important for developing Alzheimer’s treatments?
Research on microglial cells is vital for Alzheimer’s treatments because these cells influence brain health by clearing debris and supporting synaptic function. Understanding their role in disease processes can lead to novel treatment options that target the underlying mechanisms of Alzheimer’s, improving outcomes for patients.
What impact does Beth Stevens’ research have on the future of Alzheimer’s disease treatment?
Beth Stevens’ research contributes significantly to the future of Alzheimer’s disease treatment by revealing how microglial dysfunction can exacerbate the condition. Her discoveries promise to inform the development of new therapeutic strategies and early detection methods, ultimately improving care for millions affected by Alzheimer’s.
Can you explain the significance of early biomarkers identified through Alzheimer’s disease research?
Early biomarkers identified through Alzheimer’s disease research, particularly in studies involving microglial cells, are significant as they enable earlier diagnosis and intervention. This can lead to timely treatment that may slow progression, ultimately improving the quality of life for individuals at risk or in the early stages of Alzheimer’s.
How does basic science relate to advancements in Alzheimer’s disease research?
Basic science is foundational to advancements in Alzheimer’s disease research, as it fosters the understanding of fundamental processes, like those involving microglial cells. This knowledge can lead to unexpected discoveries that translate into clinical insights and new treatments, emphasizing the importance of creativity and curiosity in scientific exploration.
| Key Points | Details |
|---|---|
| Researcher Background | Beth Stevens leads research on microglial cells impacting Alzheimer’s disease at Boston Children’s Hospital. |
| Microglial Cells’ Role | They act as the brain’s immune system, clearing damaged cells and pruning synapses. |
| Aberrant Pruning | Improper pruning by microglia can contribute to Alzheimer’s and other neurodegenerative diseases. |
| Impact of Research | Stevens’ research aids in developing new medications and biomarkers for early disease detection. |
| Funding Sources | Stevens’ work is significantly supported by NIH and other federal agencies. |
| Future Implications | Research could potentially improve treatments for 7 million Americans living with Alzheimer’s. |
Summary
Alzheimer’s disease research has made significant strides due to the dedicated efforts of scientists like Beth Stevens, who is pioneering our understanding of microglial cells and their roles in neurodegenerative conditions. By investigating how these immune cells function in the brain, Stevens has established critical links between their activity and the onset of diseases such as Alzheimer’s. As we anticipate a doubling of Alzheimer’s cases by 2050, continued investment and curiosity in this field are essential to developing effective treatments and improving life quality for millions.